Treatment
Diagnosis
Due to a dysfunctional epidermal barrier, xerosis is common in patients with atopic dermatitis (AD). The application of moisturizers, especially after daily bathing, improves skin hydration, reduces disease severity, and decreases the need for pharmacologic agents. Moisturizers are primary therapy for AD as they have been shown to minimize erythema, fissuring, and pruritus.1,2,3 If the patient does not respond to moisturizers and good skin care, topical medications are often added to therapy.
In mild-to-moderate AD, topical agents are effective in reducing inflammation, preventing flares, and alleviating pruritus and other symptoms of AD.4 Topical medications may be used in combination as they have different mechanisms of action and address different aspects of the pathogenesis of AD. Topical agents are also often used in conjunction with systemic or phototherapy in patients with severe AD.1 Current treatment guidelines recommend the use of topical corticosteroids (TCS), topical calcineurin inhibitors (TCI), or both. Topical corticosteroids (TCS) are the mainstay of anti-inflammatory therapy in adults and children with AD. TCS’ suppress immune responses and inhibit the release of proinflammatory cytokines. TCS’ minimize acute and chronic signs and symptoms of AD, including pruritus, and are used for active inflammatory disease and the prevention of relapse. It is suggested that once- to twice-daily application of TCS’ in commonly flaring areas may prevent relapses. The choice and strength of TCS chosen is not established based on clinical evidence and usually depends on clinical judgment. Additional concerns with TCS use include a lack of universal standard for quantity of application, higher absorption in children due to a proportionally greater body surface area to weight ratio, and the risk of systemic absorption in areas with thin skin.1,2
Topical calcineurin inhibitors (TCI) are a class of anti-inflammatory drugs that inhibit calcineurin-dependent T-cell activation, thereby blocking the production of proinflammatory cytokines and mediators responsible for the AD inflammatory reaction. There are 2 available TCIs: tacrolimus for moderate-to-severe disease and pimecrolimus for mild-to-moderate AD. TCIs are approved as second-line therapy for short-term, noncontinuous chronic treatment of AD in non-immunocompromised individuals who failed to respond to other topical AD medications.1,2 Unlike TCS’, TCIs do not cause cutaneous atrophy or affect collagen synthesis or skin thickness. TCIs may be used as steroid-sparing agents or in areas with thin skin. The most common side effects with TCI use are local stinging and burning upon application. These side effects tend to lessen after several applications or when preceded by a short period of TCS use. Patients should be warned of this side effect to prevent premature discontinuation. TCIs should not be used on infected lesions. Boxed warnings should be discussed before patient use, such as the controversial risk of malignancy (lymphoma and skin cancer) reported in patients using TCIs.1,2
Figure 1: Step-care management of AD2
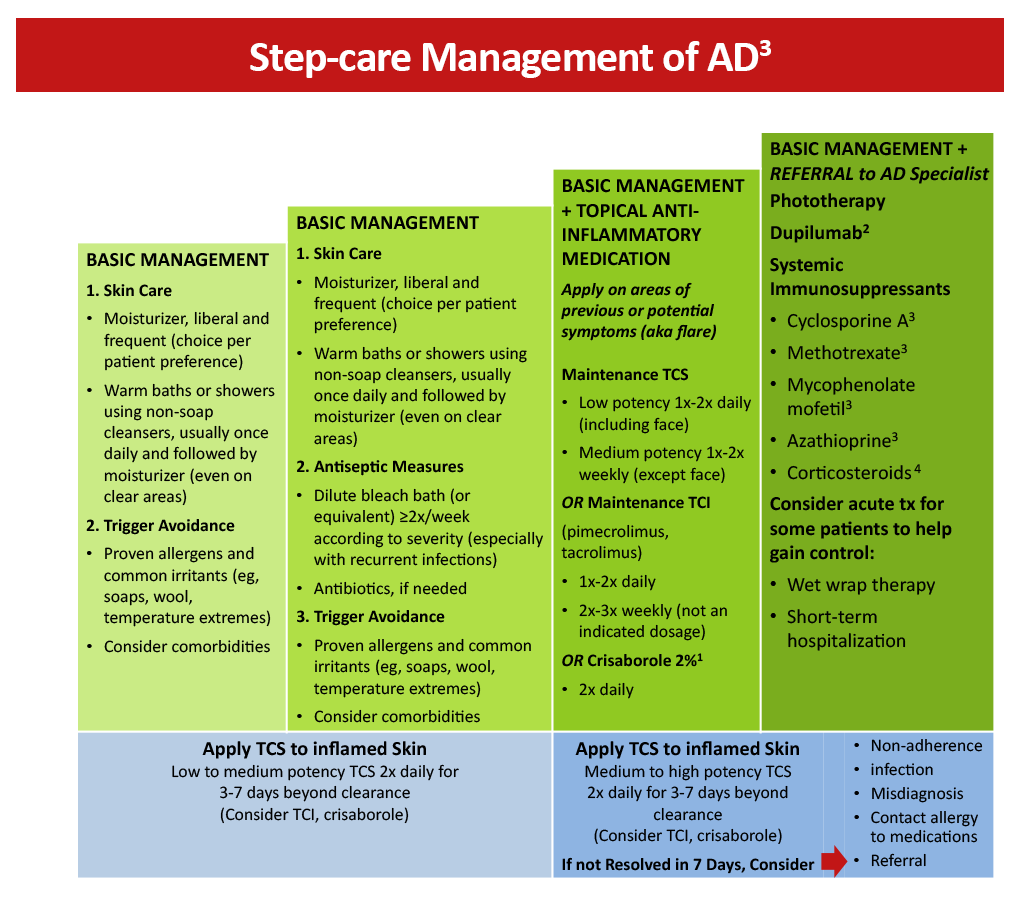
Crisaborole 2% topical ointment is a nonsteroidal anti-inflammatory phosphodiesterase 4 (PDE4) inhibitor approved to treat mild-to-moderate AD in patients aged 3 months and older. Inhibition of PDE4 has been shown to decrease the production of cytokines and significantly alleviate pruritus. Based on safety and efficacy profiles, it is suggested that TCS be used for the treatment of symptom exacerbations, followed by long-term maintenance therapy with a lower dose of TCS and/or a TCI or crisaborole. Crisaborole may be used as a first-line agent in patients who cannot use TCS or TCI based on their established adverse event profile.3
Because of the chronic nature of AD, it is important that treatment options for long-term management of the disease are safe for prolonged use. Although topical treatment options are effective in minimizing the symptoms of AD, they may be associated with application site reactions and safety concerns with use over extended periods. Emollients and topical anti-inflammatory agents are often insufficient for individuals with moderate-to-severe disease.1,5 Systemic immunosuppressants may be prescribed but are off-label in many countries and are associated with considerable adverse effects that limit treatment duration.5,6
Figure 2: AD Yardstick treatment algorithm3
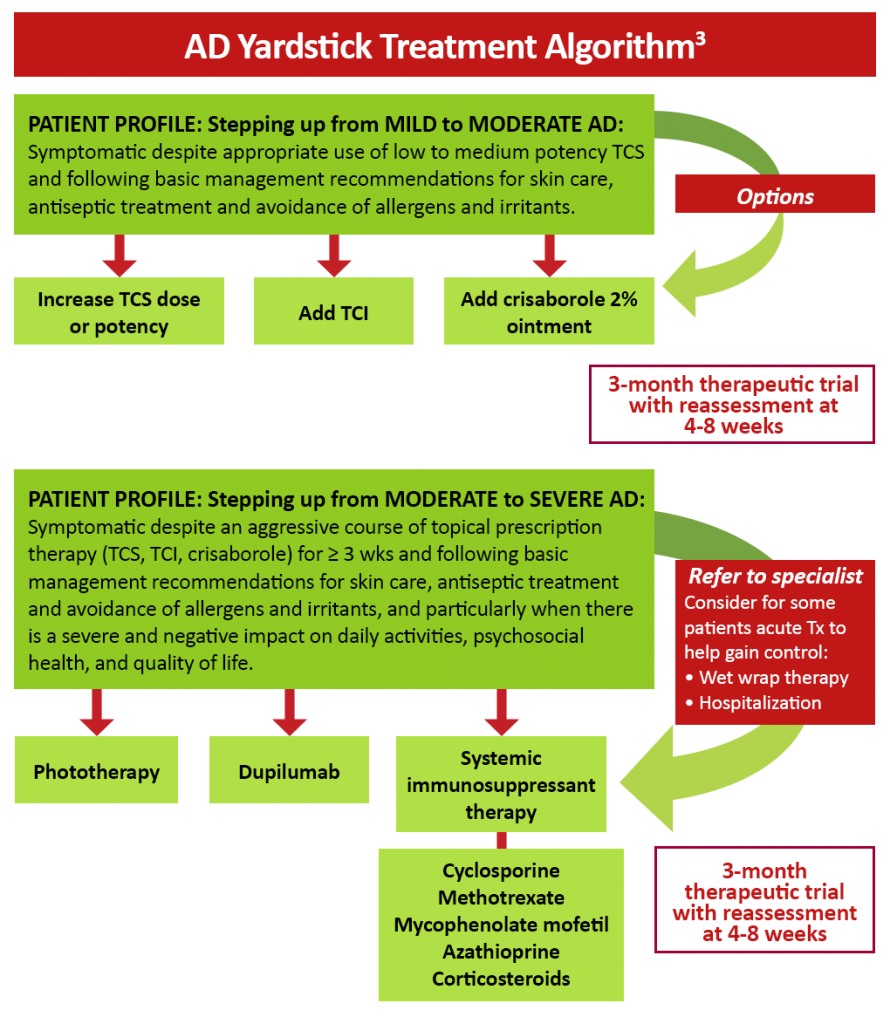
The human monoclonal antibody dupilumab has been found to be effective in reducing the severity of moderate-to-severe AD in both adult and pediatric patients by targeting Th2 cell-mediated atopic immune responses.7,8,9 Dupilumab is a fully human monoclonal antibody directed against the IL-4 receptor α subunit that is able to block the signaling of the key Th2 cytokines IL-4 and IL-13.10 Dupilumab is FDA-approved for the treatment of patients aged 6 months and older with moderate-to-severe AD whose disease is not adequately controlled with topical therapies. Additionally, dupilumab is approved for use in other atopic conditions, including moderate-to-severe asthma with eosinophilic phenotype and oral corticosteroid-dependent asthma in patients aged 6 years and older, and as add-on maintenance therapy for adults with inadequately controlled chronic rhinosinusitis with nasal polyposis.11
A brief summary of important clinical trials with dupilumab can be found below:
- In the SOLO 1 and SOLO 2 trials, the efficacy and safety of dupilumab was assessed in 671 adults with moderate-to-severe AD whose disease was inadequately controlled by topical treatment. In SOLO 1, a significant proportion of patients achieved a score of 0 or 1 on the IGA with dupilumab weekly and every other week compared to placebo (38% and 37% vs 10%; P< .001 for both regimens). Similar results were obtained in the SOLO 2 trial (36% and 36% vs 8%; P< .001 for both regimens). Dupilumab use was also associated with an improvement in QoL and a reduction in pruritus and symptoms of anxiety or depression.7
- The LIBERTY AD CHRONOS study found that the addition of dupilumab to standard topical corticosteroid therapy for 1 year significantly improved the signs and symptoms of AD in adults with moderate-to-severe AD and inadequate response to topical corticosteroids (TCS). At week 16, a significantly greater number of patients in the dupilumab + TCS groups achieved IGA 0 or 1 compared to placebo + TCS (39% for both dupilumab groups vs 12%; P< .0001). EASI-75 was also achieved in a significantly greater number of patients receiving dupilumab + TCS weekly or every 2 weeks vs placebo + TCS (64% and 69% vs 23%, respectively; P< .0001).12
- The LIBERTY AD CAFÉ trial investigated the efficacy and safety of dupilumab with concomitant topical corticosteroids in adults with AD who were inadequately controlled with cyclosporine A (CsA) or who were intolerant to or unable to take CsA. A significant improvement from baseline in EASI-75 was observed with dupilumab weekly (59.1%) and every 2 weeks (62.6%) compared to placebo (29.6%) (both regimens, P< .001). Dupilumab use was also significantly associated with improvement in QoL and AD symptoms, including pruritus, pain, sleep disturbances, anxiety, and depression.13
- Dupilumab significantly improved AD signs, symptoms, and QoL in 251 adolescents with uncontrolled moderate to severe AD who were inadequately controlled with topical medications or for whom topical therapy was not advised. A significantly higher proportion of patients reached EASI-75 at week 16 in the dupilumab every 2 weeks (41.5%) and every 4 weeks (38.1%) groups compared to placebo (8.2%) (both regimens, P< .001).8
- In children aged 6-11 years with severe AD inadequately controlled with topical therapies, Q4W and Q2W dupilumab plus TCS regimens resulted in statistically significant improvements in the signs, symptoms, and QoL of AD versus placebo plus TCS. A score of 0/1 on the IGA was achieved by 32.8%/29.5%/11.4% of patients in the Q4W/Q2W/placebo groups, respectively.9
- A phase 2 LIBERTY AD PRE-SCHOOL trial in children between 6 months and 6 years with severe AD found that dupilumab was generally well-tolerated and substantially reduced clinical signs and symptoms of AD. At week 3, treatment with 3 and 6 mg/kg dupilumab reduced mean EASI scores by -44.6% and -49.7%, respectively, in children >2 years to <6 years, and by -42.7% and -38.8% in children >6 months to <2 years.14
- In the LIBERTY AD PED-OLE, open label extension study patients enrolled under the original study protocol. Following protocol amendment, patients were switched to subcutaneous dupilumab 300 mg every 4 weeks (q4w) irrespective of weight, and newly enrolled patients were started on dupilumab 300 mg q4w. By week 52, 29.4% of patients had clear/ almost clear skin sustained for 12 weeks and had stopped medication; 56.7% relapsed and were subsequently re-initiated on treatment, with a mean time to re-initiation of 17.5 (±standard deviation 17.3) weeks.15
References
- Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: A comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4 suppl):S49-S57. https://doi.org/10.1016/j.jaci.2017.01.009
- Schneider L, Tilles S, Lio P, et al. Atopic dermatitis: A practice parameter update 2012. J Allergy Clin Immunol. 2013;131:295-299.e27. https://doi.org./10.1016/j.jaci.2012.12.672
- Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: Practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10-22.e2. https://doi.org/10.1016/j.anai.2017.10.039
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6. https://doi.org/10.1016/j.jaad.2016.05.046
- Boguniewicz M, Alexis AF, Beck LA, et al. Expert perspectives on management of moderate-to-severe atopic dermatitis: A multidisciplinary consensus addressing current and emerging therapies. J Allergy Clin Immunol Pract. 2017;5:1519-1531. https://doi.org./10.1016/j.jaip.2017.08.005
- Sidbury R, Davis DM, Cohen DE, et al. American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: Section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. https://doi.org/10.1016/j.jaad.2014.03.030
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. https://doi.org/10.1056/NEJMoa1610020
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis. JAMA Dermatol. 2020;156:44-56. https://doi.org/10.1001/jamadermatol.2019.3336
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83(5):1282-1293. https://doi.org/10.1016/j.jaad.2020.06.054
- Hamilton JD, Ungar B, Guttman-Yassky E. Drug evaluation review: Dupilumab in atopic dermatitis. Immunotherapy. 2015;7:1043-1058. https://doi.org/10.2217/imt.15.69
- Dupixent® (dupilumab) Prescribing information. Regeneron Pharmaceuticals, Inc; 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. https://doi:10.1016/S0140-6736(17)31191-1
- de Bruin-Weller M, Thaçi D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: A placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018;178:1083-1101. https://doi.org/10.1111/bjd.16156
- Paller AS, Siegfried EC, Simpson EL, et al. A phase 2, open-label study of single-dose dupilumab in children aged 6 months to <6 years with severe uncontrolled atopic dermatitis: Pharmacokinetics, safety and efficacy. J Eur Acad Dermatol Venereol. 2021;35:464-475. https://doi.org/10.1111/jdv.16928
- Blauvelt A, Gutman-Yassky E, Paller A, et al. Long‑term efficacy and safety of dupilumab in adolescents with moderate‑to‑severe atopic dermatitis: Results through week 52 from a phase III open‑label extension trial (LIBERTY AD PED‑OLE). Am J Clin Dermatol. 2022;23:365-383. https://doi.org/10.1007/s40257-022-00683-2